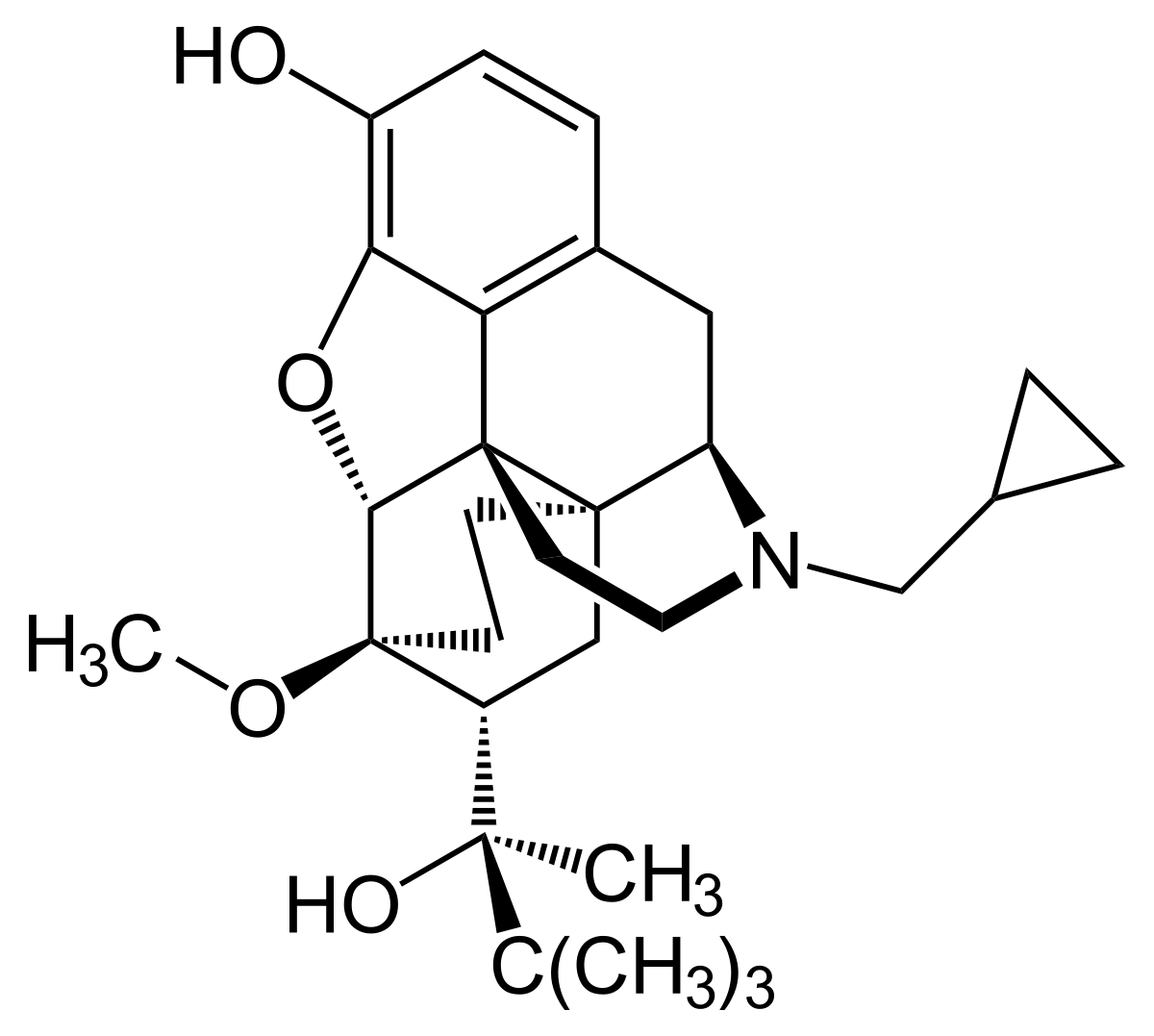
US9308202B2 - Transdermal therapeutic system for administering the active substance buprenorphine - Google Patents
Butrans (buprenorphine) is an opioid drug used to treat pain. used as a trans dermal patch Stock Photo - Alamy

DE102005016460A1 - Spriocyclic cyclohexane derivatives for the treatment of substance dependence - Google Patents

DE102006019293A1 - Transdermal therapeutic system, useful for pain treatment, comprises a carrier layer, an adhesive layer comprising a pressure sensitive copolymer with a content of fentanyl and analogs and a removable protection